Medical Devices Surface Active Coatings Market 2026–2036: USD 4.4 Billion Growth Driven by Device Adoption
Global medical devices surface active coatings market to reach USD 4.4 Billion by 2036, driven by implants, catheters, and procedural adoption.
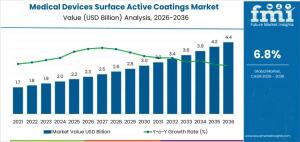
NEWARK, DE, UNITED STATES, January 17, 2026 /EINPresswire.com/ -- The Medical Devices Surface Active Coatings Market is projected to grow from USD 2.3 billion in 2026 to USD 4.4 billion by 2036, reflecting a 6.8% CAGR. The growth is primarily fueled by cardiovascular implants, orthopedic devices, and minimally invasive surgical tools, where coatings improve biocompatibility, reduce thrombosis, and enhance device longevity. Adoption is largely guided by regulatory compliance, infection prevention, and device performance rather than aesthetics. Hospitals and manufacturers evaluate adhesion, durability, and sterilization tolerance to ensure reliable device outcomes.
Request For Sample Report | Customize Report | Purchase Full Report: https://www.futuremarketinsights.com/reports/sample/rep-gb-31397
Key Market Growth Drivers
Market expansion is not driven by marketing campaigns but by regulatory compliance, validated performance, and hospital adoption. Device coatings are selected during validation and remain consistent across production cycles, ensuring quality and reproducibility. Manufacturers align surface preparation, coating deposition, and curing with device assembly schedules, while quality teams monitor adhesion, surface integrity, and regulatory documentation.
Key factors driving the market include:
- Regulatory Compliance: Coatings require full validation and approval before use across device families.
- Device Performance: Reduce friction, prevent biofilm formation, and improve wear resistance.
- Hospital Adoption: Focused on coating uniformity, handling safety, and shelf-life.
- Supplier Selection: Reliability, technical support, and traceability outweigh pricing considerations.
Product Type Insights
The market is segmented by coating type, each with distinct technical and regulatory requirements. Antimicrobial coatings lead due to their ability to reduce infections in high-risk devices. Hydrophilic coatings improve maneuverability and insertion performance, especially for implants. Drug-eluting coatings enable local therapeutic delivery, requiring rigorous stability and validation.
Product type highlights:
Antimicrobial Coatings (~46% of market): Reduce device-related infections, particularly in catheters and guidewires.
Hydrophilic Coatings: Enhance device maneuverability; small formulation changes require tight supplier collaboration.
Drug-Eluting Coatings: Support controlled therapeutic delivery; regulatory validation is critical.
These product types determine supplier engagement, quality control intensity, and production planning. Once approved, switching coatings is limited, ensuring recurring demand aligned with device pipelines.
Application Segmentation and Regional Trends
Applications with the highest volume and technical requirements are catheters, guidewires, implants, and surgical instruments. Catheters and guidewires represent ~56% of demand due to high procedural frequency and infection risk. Implants require coatings ensuring durability and biocompatibility. Surgical instruments leverage drug-eluting or lubricious coatings, emphasizing precision and controlled release.
Regional growth insights:
- India: 10.4% CAGR – hospital network expansion and device standardization.
- China: 10.2% CAGR – domestic production scale-up and hospital modernization.
- Brazil: 9.7% CAGR – increased use in implantables and surgical instruments.
- USA: 9.2% CAGR – replacement of legacy devices and adoption of advanced coatings.
- Germany: 7.4% CAGR – mature market with steady innovation uptake.
These regions demonstrate that growth is not uniform; it is driven by hospital expansions, regulatory guidance, and adoption of high-specification devices.
Regulatory and Clinical Drivers
Regulatory compliance, infection control protocols, and device standardization are key factors driving the adoption of surface coatings. Coatings affect blood contact, microbial adhesion, and wear resistance, directly impacting device performance and clinical outcomes. Hospitals standardize coatings across multiple devices to ensure consistency and repeatable clinical results.
Clinical and regulatory factors include:
- Coating consistency and adhesion strength across production lots.
- Integration with validated device assembly and sterilization protocols.
- Supplier reliability, including technical support, documentation, and training.
- Recurring demand in high-volume products like catheters, implants, and surgical instruments.
Competitive Landscape
Key players in the market are SurModics, DSM Biomedical, Biocoat, Hydromer, and AST Products. Competition focuses on process reproducibility, regulatory compliance, and delivery reliability rather than pricing. Suppliers that provide scalable production, technical support, and documentation maintain long-term positions in device pipelines.
Company highlights:
- SurModics: Hydrophilic coatings for catheters and stents with clinical data backing.
- DSM Biomedical: Polymer chemistries for cardiovascular and orthopedic implants.
- Biocoat: Anti-thrombogenic and lubricious layers for small-bore devices.
- Hydromer: Custom formulations for combination products and controlled release.
- AST Products: Contract coating services for multiple medical platforms.
Conclusion
The medical devices surface active coatings market is projected to grow steadily from USD 2.3 billion in 2026 to USD 4.4 billion by 2036 at a 6.8% CAGR. Growth is driven by regulatory compliance, device adoption, hospital network expansion, and standardized device platforms. Coatings are critical for patient safety, device performance, and manufacturing reproducibility rather than optional enhancements.
Related Reports:
Plasmid Purification Market- https://www.futuremarketinsights.com/reports/plasmid-purification-market
Orthopedic Digit Implants Market- https://www.futuremarketinsights.com/reports/orthopedic-digit-implants-market
Fill Finish Manufacturing Market- https://www.futuremarketinsights.com/reports/fill-finish-manufacturing-market
Have a specific Requirements and Need Assistant on Report Pricing or Limited Budget please contact us - sales@futuremarketinsights.com
About Future Market Insights (FMI)
Future Market Insights, Inc. (FMI) is an ESOMAR-certified, ISO 9001:2015 market research and consulting organization, trusted by Fortune 500 clients and global enterprises. With operations in the U.S., UK, India, and Dubai, FMI provides data-backed insights and strategic intelligence across 30+ industries and 1200 markets worldwide.
Contact Us:
Future Market Insights Inc.
Christiana Corporate, 200 Continental Drive,
Suite 401, Newark, Delaware - 19713, USA
T: +1-347-918-3531
Why FMI: https://www.futuremarketinsights.com/why-fmi
For Sales Enquiries: sales@futuremarketinsights.com
Website: https://www.futuremarketinsights.com
LinkedIn| Twitter| Blogs | YouTube
Sudip Saha
Future Market Insights Inc.
+1 347-918-3531
email us here
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.