Revolutionary Science Meets Entrepreneurial Excellence
Palisades Therapeutics, The Virtual Pharma Model Redefining Drug Development-15 Years of Breakthrough Innovation Without the Overhead
The platform is based on a well-defined supply line and proven technology that has been demonstrated at commercial scale. Our technology can be rapidly deployed for commercialization.”
CLIFFSIDE PARK, NJ, UNITED STATES, September 4, 2025 /EINPresswire.com/ -- In an industry where walls are built faster than barriers are broken, Pop Test Oncology LLC operating as Palisades Therapeutics has pioneered a model that is reshaping pharmaceutical R&D. For 15 years, our unique brain trust platform through our collaborators, has generated over $50 million in non-dilutive funding with zero salaried employees—demonstrating that the future of drug development lies not in buildings, but brilliant minds working in concert. — James Bruno, PhD, CMC Lead
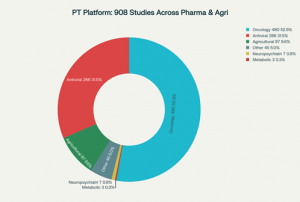
Unprecedented Research Scale: 908 Studies Across Pharmaceutical and Agricultural Markets
With 908 studies completed, our PT platform stands as one of the most extensively researched, multi-market initiatives spanning: oncology, immunotherapy, host directed antivirals, neuropsychiatric and metabolic disorders, and agricultural biotechnology. The scale and versatility align with what pharmaceutical companies seek in high-value acquisition and partnership opportunities.
The Power of Collective Intelligence
Our network of 100+ leading scientists, physicians, clinicians, lawyers, and professionals represent what the industry needs most: agility without bureaucracy, and expertise without overhead. These world-class colleagues maintain their primary positions while contributing their specialized knowledge fueling a dynamic ecosystem that traditional pharmaceutical companies struggle to match.
This approach mirrors 2025's pharmaceutical trends: 77% of pharma executives expect increased merger and acquisition (M&A) activity with growing interest in virtual pharma partnerships that accelerate innovation while minimizing risk.
As we mark 15 years of drug development dedicated to saving and transforming lives, we’re profoundly grateful to the colleagues and partners who have been with us on this journey. They include the Department of Defense, the VA, National Institutes of Health /NIAID, NCI, NIDA, NIAAA, and the USDA and great companies such as BioReperia, InVivo Bio, RTI, Leidos, Sai Life Science, TPM Labs and TGEN Drug Development(TD2). And we are equally proud to have worked alongside brilliant research teams at top institutions across the U.S., Asia, and Europe—Colorado State University, University of Kentucky, Mt. Sinai-Icahn School of Medicine, Baylor College of Medicine, Kurume University School of Medicine, Georgetown University Medical Center, Harvard University, IrsiCaixa (Barcelona), Utah State University, Southern Research, Medical University of South Carolina, University of Manchester, Central State University, and many more—we are proud to stand on the shoulders of true innovators pushing medicine forward.
Our platform delivers pharmaceutical-grade rigor across seven therapeutic candidates
Cancer Research Excellence
Our 480 NCI-60 cancer cell line screens represent the gold standard that has underpinned FDA approvals in oncology screening. The National Cancer Institute’s NCI-60 panel, recognized as "the most important step forward in cancer drug screening", has contributed to breakthrough approvals including Bortezomib and Eribulin. Our comprehensive screening across four PT compounds positions us advantageously in the $200+ billion global oncology market.
Host Directed Antiviral Research Innovation
286 viral testing studies across 17 virus families underscore our leadership in a $40+billion antiviral market, where alliances like Harvard-AbbVie's ($30M) and Gilead-Assembly Bio's 12-year partnership, highlight the demand.
PT150 : Breakthrough AUD Therapy with Validated Clinical Safety
A 2025 Journal of Addiction Medicine publication validates its safety and efficacy in treating alcohol use disorder (AUD), positioning us to capture a massive underserved market.
Clinical Validation Results from 2025 Study
• No significant drug-drug interactions when co-administered with alcohol
• No serious adverse events in Phase I trials
• Well-tolerated safety profile supporting clinical advancement
PT157 Dimer Technology: Next Generation IP Protection
Our strategic pairing of PT150 (monomer) and PT157 (dimer), strengthens both efficacy and creating comprehensive intellectual property protection across multiple therapeutic areas.
Advantages of PT157 Dimer Technology
• Higher potency at lower doses (better compliance, and lower cost)
• Extended duration of action (fewer doses, improved outcomes)
• Stronger receptor binding (thermodynamic advantage)
Comprehensive Patent Protection
• Composition of matter and use patents across multiple therapeutic areas
• Broad claims covering structural variations for robust IP protection
Dimer strategies have proven transformative in drug development, with dimeric compounds showing higher activity than monomeric units and enabling stronger biological responses. Our PT157 dimer technology positions us advantageously in licensing negotiations and acquisition scenarios. According to James Bruno, PhD, our CMC Lead, “The platform is based on a well-defined supply line and proven technology that has been demonstrated at commercial scale. Our technology can be rapidly deployed for commercialization.”
Agricultural Innovation: Expanding Platform Value into $380B Market
Our platform also extends into the $167B+ global agricultural biotechnology market(projected $384 billion by 2032 at a 10.9% CAGR.) Agricultural research distribution shows PT150 and PT159 leading the platform's agricultural innovation with comprehensive pest and pathogen control studies.
• Citrus Greening Disease (HLB): Our compounds address one of agriculture's most devastating problems, which has caused $4.5 billion in losses in Florida alone and eliminated 52.6 million trees in Brazil. With 16 specialized HLB control studies, our PT159 compound achieves 100% inhibition of causative bacteria at ultra-low concentrations.
• Dual-Action Innovation(PT159 & PT160): Simultaneously target insect vectors and bacteria pathogens an innovation valued in the $92B+ crop protection market.
• TPR1 Leads USDA’s National Citrus Greening Compound Evaluation-Advances to Phase 2 Grove Trials
Investment-Ready Platform with Multi-Market Value
Our virtual pharma model is uniquely positioned to address looming industry headwinds:
• $300 billion in pharma revenue face patent cliffs by 2030
• Companies seek non-dilutive funding partnerships and validated clinical assets
Key Advantages:
• $50M+ non-dilutive research investment
• 908-study regulatory-grade database
• Clinical-stage validation (human safety and efficacy data)
• Strategic patent portfolio(monomer+ dimer IP)
• Scalability across $500B+ therapeutic and $380Bn agricultural biotechnology markets
Partnership Opportunities : Pharmaceutical and Agricultural
• Licensing
• Strategic Partnerships
• Acquisition pathways
The critical difference between Palisades Therapeutics’ compounds and traditional treatments on the market is the ability to deliver breakthrough results with lower chemical burden and enhanced therapeutic precision.
Let’s talk about how our virtual pharma model, 15-year milepost, can accelerate your pipeline while delivering compelling risk-adjusted returns.
@AbbVie @Johnson-Johnson @Alkermes @Bristol-Myers-Squibb @Astellas-Pharma @Pfizer @Gilead @Regeneron @Ono-Pharma
#Biotech #Addiction #PharmaNews #ClinicalTrials #Breakthrough#AlcoholUseDisorder #AgricultureBiotech #BiotechPartnerships #DrugDevelopment #TherapeuticPlatform #PharmaM&A #OncologyResearch #CitrusGreening
Forward-looking statements regarding business prospects are based on current expectations and involve risks and uncertainties that could cause actual results to differ from those expressed or implied.
Randi Altschul
Pop Test Oncology/Palisades Therapeutics
randi@poptestllc.com
Visit us on social media:
LinkedIn
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.